近日,卫生毒理学研究团队成功构建了AHR激活为始发分子时间的不良结局路径 (Adverse Outcome Pathway, AOP),相关研究成果以“A toxicity pathway-oriented approach to develop adverse outcome pathway: AHR activation as a case study”为题,在线发表在期刊Environmental Pollution (Impact Factor: 6.792) 上 (DOI: https://doi.org/10.1016/j.envpol.2020.115733)。
进入市场和商业化的化学物日新月异可能给环境和人类健康带来潜在危害。对这些化学物进行及时、准确的风险评估是一项巨大挑战。 构建不良结果途径 (AOP)框架有望更好地系统性理解外源环境化学物的毒性机制、从而有助于加快风险评估过程。 为了通过AOP方法探索环境化学物质的毒性机理,首选将充分的实验数据与系统生物学知识相结合。本研究中,开创性地建立了一种可靠且合理的AOP新方法。首先,收集、整合从比较毒理基因组数据库 (CTD) 获得的化学物–基因互作关系,毒性通路分析,分子调控,表型等信息,进而结合IPA (Ingenuity Pathway Analysis) 进行分析,最后构建AOP 网络框架。 本研究以经典毒物苯并(a)芘 (BaP) 为例,确定其关键的毒性通路,分子起始事件 (MIE) 和关键事件 (KEs) 来分别构建肝和肺中的AOP。 此外,本研究还使用了包括2,3,7,8-四氯二苯并对二恶英 (TCDD),丙戊酸,槲皮素和PM等多个典型AHR配体来验证此AOP网络。
系统毒理学是指通过整合分子、细胞、组织等多个不同层次的高通量信息,系统研究外源化学物或环境应激因素与基体相互作用的一门学科。AOP是系统毒理学的毒效动力学方向的热点话题,旨在通过利用体外毒性测试实验简化化学物质的毒性评价流程,使得从毒作用机制触发的化学物风险管理策略成为可能。本研究的AOP构建方法和互作网络模型可为相关研究带来新的见解。
于典科教授为本文的通讯作者,靳远老师和硕士研究生冯美瑶、马万里为本文的共同第一作者。
上述研究得到国家重点研发计划项目(2017YFC1600201)、国家自然科学基金(91743113、91943301、81973075)和山东省泰山青年学者基金(tsqn201812046)的支持。
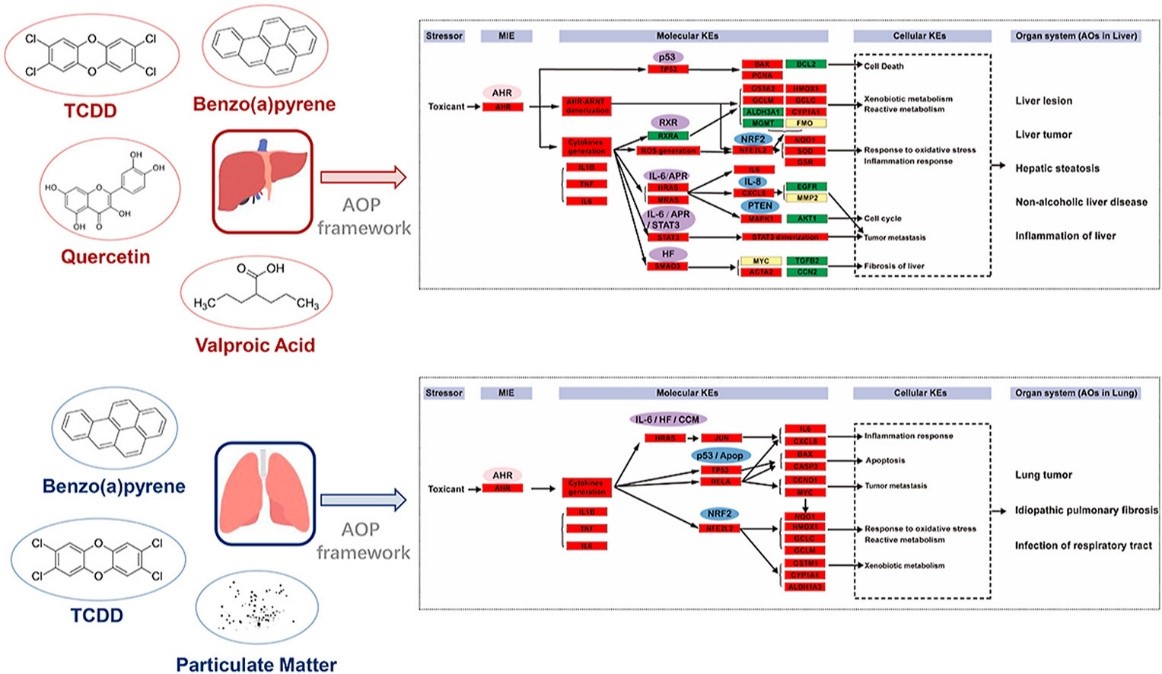
图:AHR激活引发的肝/肺毒性的AOP(图片来自DOI: 10.1016/j.envpol.2020.115733)
Recently, Dr. Dianke Yu’s lab of Toxicology in the School of Public Health has proposed a novel AOP developing method based on toxicity pathways and constructed the AOPs describing AHR activation-initiated liver/lung toxicity. Relevant results have been published in Environmental Pollution (Impact Factor: 6.792) (DOI: https://doi.org/10.1016/j.envpol.2020.115733) under the title of “A toxicity pathway-oriented approach to develop adverse outcome pathway: AHR activation as a case study”.
With numerous new chemicals introduced into the environment everyday, identification of their potential hazards to the environment and human health is a considerable challenge. Developing adverse outcome pathway (AOP) framework is promising in helping to achieve this goal as it can bring In Vitro testing into toxicity measurement and understanding. To explore the toxic mechanism underlying environmental chemicals via the AOP approach, an integration of adequate experimental data with systems biology understanding is preferred. Here, we describe a novel method to develop reliable and sensible AOPs that relies on chemical-gene interactions, toxicity pathways, molecular regulations, phenotypes, and outcomes information obtained from comparative toxicogenomics database (CTD) and Ingenuity Pathway Analysis (IPA). Using Benzo(a)pyrene (BaP), a highly studied chemical as a stressor, we identified the pivotal IPA toxicity pathways, the molecular initiating event (MIE), and candidate key events (KEs) to structure AOPs in the liver and lung, respectively. Further, we used multiple typical AHR-ligands, including 2,3,7,8-tetrachlorodibenzoparadioxin (TCDD), valproic acid, quercetin, and particulate matter, to validate our AOP networks.
Our approach is likely to speed up AOP development as providing a time- and cost-efficient way to collect all fragmented bioinformation in published studies. It also facilitates a better understanding of the toxic mechanism of environmental chemicals, and potentially brings new insights into the screening of critical paths in the AOP network.
Dr. Dianke Yu is the corresponding author of the paper. Dr. Yun Jin and postgraduate students Meiyao Feng, Wanli Ma are the co-first authors.
This study was supported and funded by the National Key R&D Program of China (2017YFC1600201), National Natural Science Foundation of China (Grant No. 91743113, 91943301, and 81973075), and Young Taishan Scholars Program of Shandong Province (Grant No. tsqn201812046).

Figure: AOPs describing AHR activation-initiated liver/lung toxicity. (DOI: 10.1016/j.envpol.2020.115733)